1. Scope of Coverage
These guidelines are for any domestic or international academic/non-profit biomedical research body or private company in the biotech/pharmaceutical industries who, in order to conduct research in the biomedical sciences, is interested in obtaining biological samples, data or information from MJ Health Resource Center (hereinafter referred to as the Center).
In accordance with the Human Biobank Management Act Article 15, the Center’s biological samples (derivatives aside) may not be exported. Furthermore, any international transport of data or sample derivatives from the Center requires prior approval from the Competent Authority.
2. Application Rules
2.1 Applicant Qualifications
In applying for access to Center samples or data, all research institutions and corporations must provide qualification documentation.
2.2 Data
2.2.1 Upon completion of the evaluation and approval process, the Center provides de-identified biological samples, data and other associated information to the qualified applicants.
2.2.2 Biological samples will not be exported in compliance with Human Biobank Management Act. Derivatives of the biological samples or its associated information can be exported only when official approval is granted by Competent Authority.
2.2.3 The Center is responsible for ensuring the method of transport for biological samples, data or other associated information complies with Personal Information Protection Act and other associated legal guidelines.
3. Application Process
3.1 In order to apply for biological samples, data or information access, the applicant must fill out two forms provided below:
a). MJ Health Resource Center Data Access Application
b). MJ Health Resource Center Data Utilization Project Proposal Format
In addition, a project proposal must be provided containing the following items
1). Name of research body or corporation
2). Name of project manager or principal investigator
3). Project overview
4). Description of how the biological data and samples will be used
5). Project funding source(s)
6). Projected commercial profits and reciprocity plan
7). Proof of research body/company’s own IRB approval
8). Other relevant information that could facilitate the project’s approval process
3.2 Please mail the completed project proposal and the forms to the following address:
Division of General Administration
MJ Health Research Foundation MJ Health Resource Center
3F.-3, No.58, Xingshan Rd., Neihu Dist.
Taipei City 11469, Taiwan (R.O.C.)
4. Review Rules
Each application received by the Center will be evaluated based on a set of criteria listed below:
i). The project itself is related either to basic biological/biomedical research or disease prevention, while its objective corresponds with the Center’s mission of promoting health and general welfare worldwide.
ii). Former applicant please refer to past agreements with the Center and the guidelines listed therein.
iii). The project must avoid negative social or psychological impacts on the sample or data donor.
5. Review Process
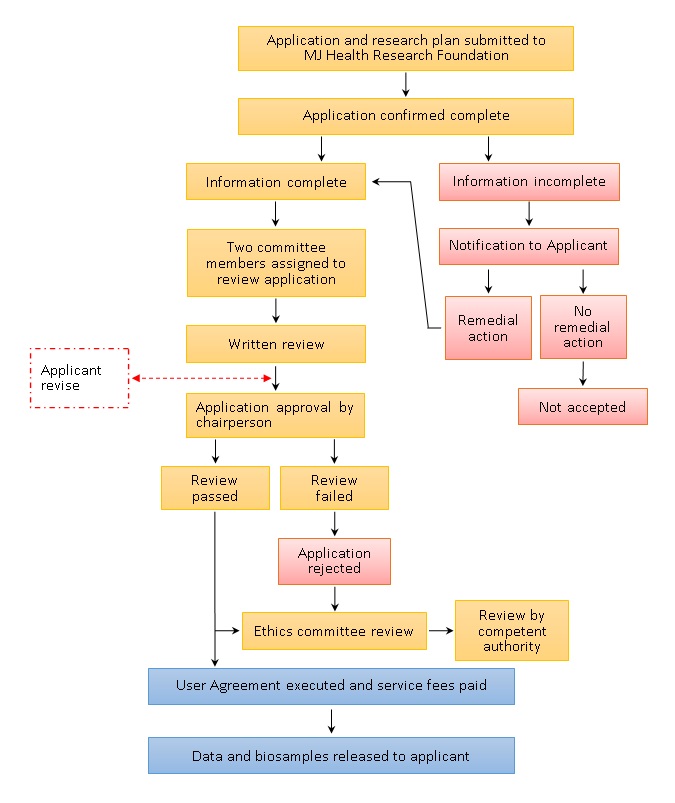
5.1 Research plan and application submission
5.1.1 The Applicant submits an application and supporting documentation following stated requirements.
5.1.2 Application and supporting documentation are received by Center staff.
5.1.3 Application and documentation completeness are confirmed.
5.1.4 If the application and/or documentation are found to be incomplete, the Applicant will be notified and asked to submit the missing information
5.2 Internal review
5.2.1 Two committee members shall be assigned by the chairperson to review the application.
5.2.2 The reviewers produce a written review and reply.
5.2.3 Once the review is complete, the Applicant will be advised of any required amendments. Finally, the review results are confirmed and approved by the chairperson.
5.2.4 If the application is accepted, the Applicant will be notified of follow up processes. Otherwise, the Applicant will be notified that the project has been rejected.
5.2.5 The chairperson may choose to submit the application to the ethics committee for review.
5.2.6 Following review, the ethics committee will file a report with the Competent Authority.
5.3 Service fee payment process
5.3.1 The Applicant executes the MJ Health Resource Center Biological Samples, Data and Information User Agreement (“User Agreement”).
5.3.2 Upon application approval, the Applicant will be notified to pay the service fee.
5.3.3 Once the service fee is received, the samples and data will be released to the Applicant.
Please refer to the following flowchart

6. Material Transfer
6.1 Signing the User Agreement and Paying the Management/Service Fees
6.1.2 Management/Service Fees
In order to cover costs incurred due to biological samples and data management or storage, the Center will collect Management/Service Fees from the approved applicant according to the published fee schedule.
6.2 Once the User Agreement has been signed and payment for the Management/Service Fees has been received, material transfer will commence.
6.3 Material Transfer Methods
Once the application for biological data and information is approved, the Center will prepare the dataset according to the applicant’s technical specifications, de-identify the data, transfer them to a CD/DVD-ROM with a unique serial number and send it to the applicant. Biological samples will also be de-identified, packaged and shipped to applicant under appropriate temperature and delivery conditions. Staff at the Division of General Administration will notify the approved applicant of details regarding shipping and handling.
7. Appropriate Use of Biological Samples and Data
7.1 Approved Use
Approved applicant shall utilize the biological samples and data only within the area specified in the approved project proposal. Material use outside the approved proposal is strictly prohibited. Should the need to use the material outside the approved area arise, applicant must re-apply to modify the existing project proposal and must be re-approved by the Ethics Committee prior to use.
7.2 Material Storage
Approved applicant shall observe the relevant laws, regulations, as well as the Center’s Information security guidelines with regard to the storage of biological samples, data and information. Applicant must also perform biological information security audit as needed in accordance with the Center’s Annual Biological Information Security Audit Plan per the legal requirements of the Human Biobank Information Security Act Article 18. Records of such audits will be kept in permanent storage.
8. Research Result Publications
When approved applicant decides to publish its research result based on the use of biological samples or data from the Center, proper citation crediting the Center’s involvement or assistance must be included in the official publication. Other necessary reference information to be included in the publication is listed under Article 4 - Research Result Publication and Disclosure in the MJ Health Resource Center Biological Samples, Data and Information User Agreement. MJ Health Research Foundation will publish a list of approved applicants and their research results in accordance with Human Biobank Management Act Article 22.
9. Agreement on the Reciprocity of Commercial Profits
9.1. Application materials must include Reciprocity Plan for Commercial Profits, which must be approved by the Center.
9.2. Applicant shall supply information on any planned commercial use of research results to the Center. Details will be included in the contract between the Center and the data user. If the data user is the Center, its Ethics Committee will determine the Reciprocity percentage and Fee amount.
9.3. In implementing the Reciprocity agreement, applicant shall supply records of relevant financial transactions and fulfill its financial obligation to the Center as agreed in the contract.
9.4. The Center shall return no less than 50% of the Reciprocity proceeds due to commercial use of research result by the applicant to the general public.
10. Damage Compensation Guidelines due to Unlawful Disclosure of Data
10.1 The Center and the applicant shall both sign the MJ Health Resource Center Biological Samples, Data and Information User Agreement, which stipulates the expected area of use for the materials obtained by the approved applicant.
10.2 If private information is revealed to the unauthorized audience, the Center shall assume legal actions in accordance with MJ Health Resource Center Guidelines for Participants’ Damage Compensation, MJ Health Resource Center Biological Samples, Data and Information User Agreement and other related regulations.
11. General Rules for Data Linkage
The Center respects the ROC government’s official policy and will perform encrypted data linking only with database approved by the government (i.e. Death Registration File). Applicant must state clearly the intents and purposes for data linking in the project proposal during the application process. Once approved by the Ethics Committee, the Center will perform data linking on behalf of the applicant. The applicant will not receive personally identifiable information from the Center in the delivered data linkage result. The Center will not perform data linking with database not yet approved by the government, including those that produce only grouped data after encrypted data linkage.